SpaceOAR Hydrogel is designed to help reduce the radiation dose delivered to the rectum during prostate cancer radiation treatment.
When treating prostate cancer patients with radiation therapy, the goal is to target the cancer cells while avoiding damage to surrounding healthy tissue.
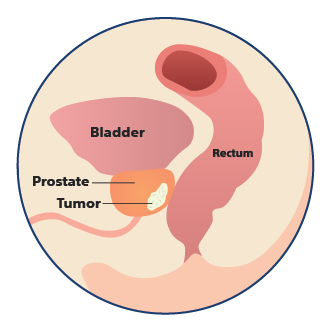
Anatomy without SpaceOAR Hydrogel
The prostate is next to the rectum and naturally separated by a small space. Due to the proximity, prostate radiation therapy can unintentionally cause damage to the rectum, which can lead to issues with bowel function.1
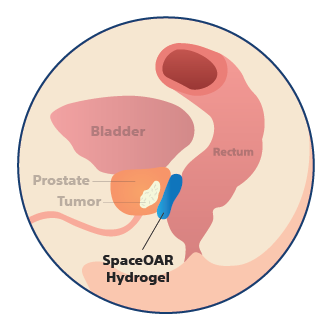
Anatomy with SpaceOAR Hydrogel
The hydrogel spacer pushes the rectum away from the prostate and is designed to reduce the radiation dose delivered to the rectum during prostate radiation therapy.
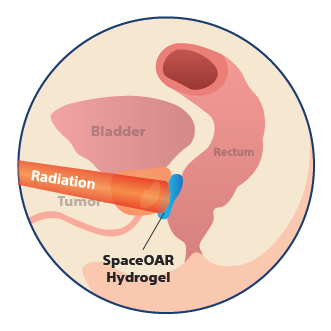
SpaceOAR Hydrogel with Radiation Therapy
By pushing the prostate farther from the rectum, the radiation dose delivered to the rectum is reduced, which may help minimize the side effects of radiation therapy.2-4
What is the Hydrogel made of?
SpaceOAR Hydrogel is an FDA-cleared medical device made mostly of water (90%) and polyethylene glycol (PEG; 7%), that when combined, form a soft gel-like synthetic material commonly used in other medical applications such as in the eye, brain, and spine.5
You should discuss any known allergies you may have to the SpaceOAR Hydrogel with your doctor.
Frequently Asked Questions
![]()
![]()
Find out more about SpaceOAR Hydrogel
![]()
![]()
![]()
SpaceOAR Hydrogel is intended to temporarily move the rectal wall away from the prostate during the course of radiotherapy treatment for prostate cancer, and in creating this space it is the intent of SpaceOAR Hydrogel to reduce the radiation dose affecting the rectum.
SpaceOAR Hydrogel contains polyethylene glycol (PEG). As with any medical treatment, there are some risks involved with the use of SpaceOAR Hydrogel. Potential complications associated with SpaceOAR Hydrogel include, but are not limited to: pain associated with injection, pain or discomfort from the hydrogel, site inflammation, infection (including abscess), inability to urinate, urgent need to urinate, constipation, rectal muscle spasm, damage to lining of rectum, ulcers, fistula (a holebetween rectum and bladder, urethra, or skin below the scrotum), perforation (hole in prostate, bladder, urethra, rectum), necrosis (dead tissue), allergic reaction (local reaction or more severe reaction, such as anaphylaxis), embolism (blood vessel blockage is possible and may happen outside of the pelvis, potentially impacting vital organs or legs), fainting, and bleeding. Please talk to your doctor about the risks and benefits related to using SpaceOAR Hydrogel. If one or more of these complications occur, you may need medical treatment or surgery. URO-1288805-AA APR 2022
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for use only in countries with applicable health authority registrations. This material not intended for use in France. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. Please check availability with your local sales representative or customer service.
References
*Number of patients is based on units shipped and a BSC proprietary algorithm.
- Radiation Therapy for Prostate Cancer. American Cancer Society. http://www.cancer.org/cancer/prostate-cancer/treating/radiation-therapy.html. Accessed April 25, 2023.
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5):971-7.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017 Apr 1;97(5):976–85.
- Hamstra DA, Mariados N, Sylvester J, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract Radiat Oncol. 2018;8(1):e7‐e15.
- Data on file with Boston Scientific



